Viral infection as an NAD+ battlefield
Published in nature Nature Metabolism (03 January 2022)
Subject covered by this article:
“Coronavirus replication results in expenditure of nicotinamide adenine dinucleotide (NAD+), the central catalyst of cellular metabolism, in the innate response to infection. Repletion of NAD+ levels has the potential to enhance antiviral responses.” (bold added)
Quotes:
- “…From their earliest replication intermediates, coronaviruses elicit an attack on cellular NAD+. Cellular NAD+ becomes a battlefield in which the innate immune system and the virus struggle for the upper hand.” (bold added)
- “…early clinical data indicate that NAD+ repletion might constitute an antiviral treatment approach that could be generally useful against infectious agents that activate the interferon system.” (bold added)
- “…a double-blinded phase 3 trial was completed, in which patients with mild-to-moderate COVID-19 were assigned to standard of care plus placebo (n = 75) or to standard of care plus a combination of four nutritional supplements, consisting of 3.7 g carnitine, 2.6 g N-acetylcysteine, 1 g NR [Nicotinamide Riboside] and 12.4 g serine (n = 229), twice a day for two weeks. Standard of care was either hydroxychloroquine or favipiravir, the repurposed malaria and flu drugs that do not have activity against SARS-CoV-2 in clinical trials.”
- “… We also suggest prevention trials to determine whether fortification of the NAD+ system may represent a relatively inexpensive way to protect exposed healthcare workers and housemates from viral infection. (bold added)
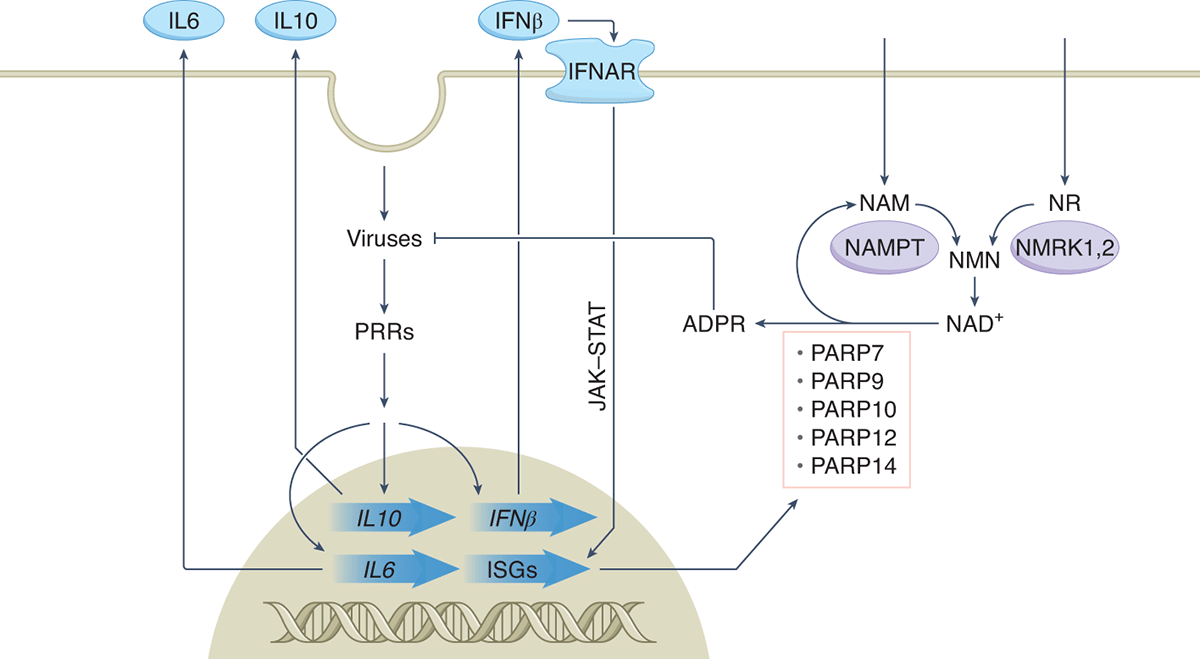
Deployment of NAD+ in antiviral innate immunity Upon viral entry, pattern recognition receptors (PRRs) launch expression of inflammatory cytokines including IL6, IL10 and IFNβ. These molecules exit cells and act on the same or neighboring cells. Engagement of IFNβ with interferon receptors (IFNAR) leads to activation of the JAK–STAT pathway and interferon-stimulated gene (ISG) transcription. Among the ISGs, five members of the PARP superfamily are transcriptionally induced concomitant with expenditure of cellular NAD+ and transcriptional upregulation of genes for nicotinamide (NAM) and nicotinamide riboside (NR) salvage. Repletion of cellular NAD+ with NR is being tested as a means to boost the antiviral activities of the PARP superfamily members and has been found to be associated with calming the cytokine storm. NAM is undergoing clinical testing as an antiviral as well. ADPR, ADPribose; NAMPT, NAM phosphoribosyltransferase; NMN, NAM mononucleotide; NMRK, NR kinase.